Navigation
Modelling Neuroblastoma's Clonal Evolution during Chemotherapy
I conceived this spin-off while working on PRIMAGE and secured an Insigneo research grant to fund it.
In the summer of 2020, I recruited Abigail Barlow, an MMath student at the University of Sheffield, to profile the drugs approved for neuroblastoma. After that, we learnt to use genetic algorithms to optimise drug combination and scheduling.
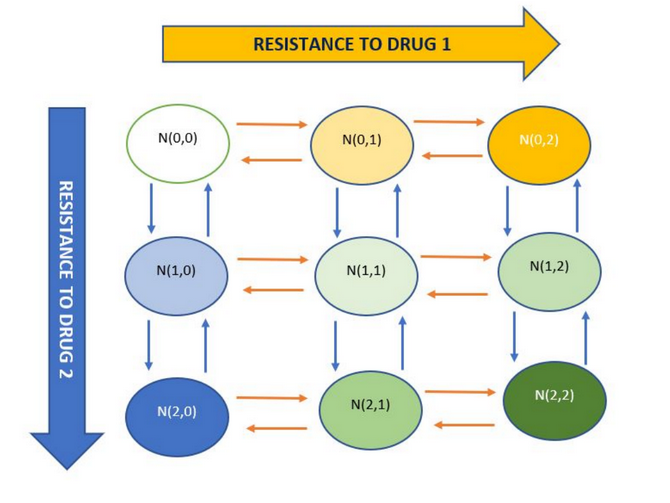
In the academic year 2020–2021, Ritvik Mehra carried out his undergraduate thesis research (computer science) at the University of Sheffield under Dr Dawn Walker's and my supervision. He used four ordinary differential equations inspired by the logistic function to model neuroblastoma's clonal evolution under the selective pressures exerted by etoposide and cisplatin. Then, he fitted the model to experimental data in the literature using the Levenberg-Marquardt algorithm and designed a genetic algorithm to optimise the combination of the two drugs.
After Ritvik's thesis research, I established international collaborations with Dr Fabio Dercole from the Polytechnic University of Milan in Italy and Dr Sabine Taschner-Mandl from St. Anna Children's Cancer Research Institute in Austria. Under our supervision, Matteo Italia, Dr Dercole's PhD student (engineering mathematics), modified and expanded Ritvik's model to consider clonal competition, mutation, pharmacokinetics, and the drugs' cytotoxic effects. In the final model, each clone has its own fitness, proliferation rate, and drug sensitivities; and it can adapt to a drug at the expense of proliferation. As he found more relevant data on vincristine and cyclophosphamide than etoposide and cisplatin, Matteo calibrated the model to describe the former pair.
Then, we used genetic and gradient descent algorithms to optimise induction chemotherapy, the first stage of the multimodal therapy for high-risk neuroblastoma patients. We discovered different evolutionary dynamics in tumours with different resistance profiles. According to the results, when two drugs are differentially cytotoxic, administering both at their maximum tolerated doses is not always the best strategy. The best strategy depends on each drug's relative cytotoxicity, each clone's sensitivity to each drug, and the precise clonal composition.
In the broader context of precision medicine for neuroblastoma, our results suggest that evolutionary dynamics are patient-specific and as a result, personalised schedules are needed to exploit clonal competition optimally to minimise the tumour size. As chemotherapy is known to enrich mutations, not only can this approach optimise induction chemotherapy, but it can also drive the tumour into an evolutionary trap and extinction vortex simultaneously. Immediately after the optimised induction phase, the remaining small and scattered populations of cancer cells will be vulnerable to therapies targeting the enriched mutations.
We finished the project in December 2022.