Navigation
A Theoretical Study of the MYCN Enigma in Neuroblastoma
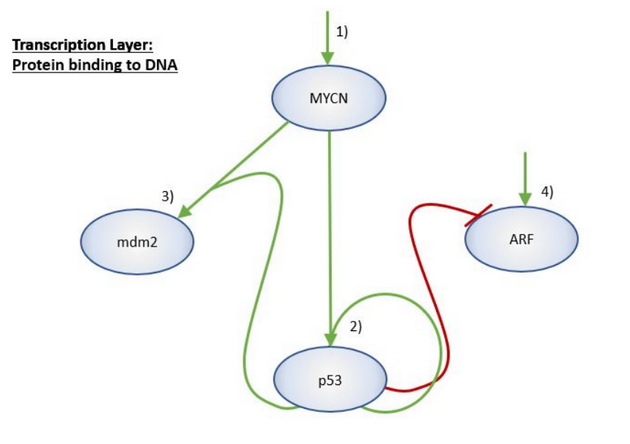
When the MYCN gene (an oncogene) is amplified in neuroblastoma cells, it is a biomarker indicative of adverse outcomes. This is not surprising because it activates genes involved in metastasis, survival, proliferation, pluripotency, self-renewal, and angiogenesis, while switching off genes involved in differentiation, cell cycle arrest, and immune surveillance. However, the abundance of MYCN's mRNA in a tumour is a non-linear prognostic indicator. The MYCN enigma is the general observation that a patient's clinical outcome does not linearly depend on whether the gene is amplified and the abundance of its mRNA in their neuroblastoma cells.
During the PRIMAGE project, I conceived this side project to find a theoretical solution to the MYCN enigma. Specifically, I wanted to develop an explanation in terms of the fact that MYCN activates p53 (a tumour suppressor gene) through the ARF/MDM2/p53 axis. In order to fund it, I secured two Insigneo research grants.
Mathematical Modelling
In the summer of 2019, I recruited Daniel Jordan, a mechatronics and robotics engineering undergraduate student, to work on the project at the University of Sheffield. Together, we used a set of ordinary differential equations to model the crosstalk between MYCN and the ARF/MDM2/p53 axis, and performed a basic analysis on the fixed points.
In the academic year 2019–2020, Melody Parker, an MSc computational medicine student at the University of Sheffield, joined the project for her dissertation research. Under the supervision of Dr Dawn Walker and me, she picked up where Dan had left off, enriching the model with mechanistic details concerning various stages in the flow of genetic information. Using eight equations containing highly non-linear terms such as the Hill function, she modelled the dynamics within a gene regulatory network representing these mechanisms.
Phase Space Analysis
In the summer of 2021, Rory Deignan, an undergraduate (theoretical physics) at the University of Sheffield, built computational tools for a large-scale stability analysis of the model. He combined Latin hypercube sampling with the Newton-Raphson algorithm to find physical and well-scaled fixed points and classify them. He also experimented with spectral and fractal analysis tools.
In 2023, I recruited Matteo Italia, a PhD candidate under Dr Fabio Dercole at the Polytechnic University of Milan. We hypothesised that the protein level of p53 relative to that of MYCN determines a neuroblastoma cell's fate. We parameterised the model with literature values before conducting a global sensitivity analysis. We partitioned the simulation results into different categories based on our hypothesis and compared each set of results with experimental datasets. We also applied the Apriori algorithm to find association rules in each set of simulation results.
Beyond the Hypothesis
The simulation results are consistent with numerous findings in the literature. Also consistent is the idea that MYCN regulates biosynthesis in neuroblastoma cells, but mutations shift the relative rates of producing MYCN and p53. A key prediction based on this idea is that MYCN and certain stress signals form a negative feedback loop.