Navigation
A Theoretical Study of Evolutionary Therapy for Neuroblastoma
Evolutionary therapy exploits the evolutionary dynamics within a tumour comprising subpopulations with different combinations of mutations. An example is adaptive therapy, which uses treatment-sensitive cancer cells to suppress their resistant peers. This project aims to investigate evolutionary therapy in the context of neuroblastoma.
Together with Dr Matishalin Patel, an expert in evolutionary biology and a departmental colleague at the University of Hull, I applied for and secured a DAIM PhD studentship after a competitive process. In January 2024, Francesca Covell became our first PhD candidate (AI and Data Science). An international collaboration with Dr Sabine Taschner-Mandl from St. Anna Children's Cancer Research Institute was established.
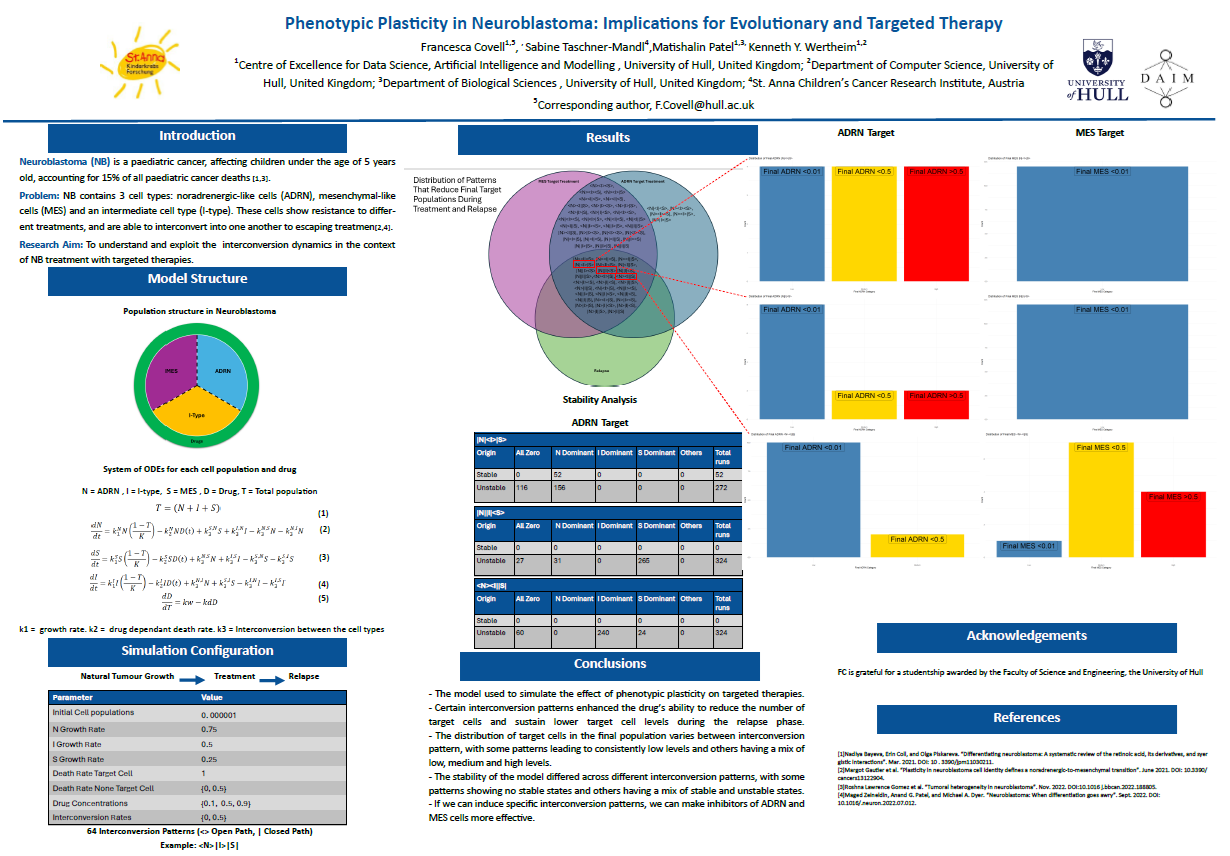
Neuroblastoma's phenotypic plasticity is an important factor in the context of evolutionary therapy. In her first year as a PhD candidate, Fran built and validated a compartmental model with three homogeneous subpopulations containing noradrenergic, intermediate, and mesenchymal neuroblastoma cells respectively. Noradrenergic neuroblastoma cells are typically more sensitive to treatment (like ALK inhibitors), but Dr Taschner-Mandl has experimented with mesenchymal phenotype–specific inhibitors. Intermediate neuroblastoma cells resemble cancer stem cells.
The model describes the interconversion dynamics between the three compartments. In her second year, Fran simulated the effects of both inhibitor types for each of the 64 possible interconversion patterns. She has generated a comprehensive dataset by solving initial value problems, thereby identifying four therapeutically useful patterns.
She is now investigating these patterns in detail by sensitivity and stability analysis.